The flame reaction
About the flame reaction
Firework draws a beautiful picture in the night sky. It is the application of the flame reaction. We can enjoy a colorful firework by mixing metal salt in the gunpowder.
When metal salt is put in the flame, the color which is characteristic of the metal is seen. This phenomenon was known experientially in the 19th century.
In the middle part of 19 centuries, chemist's Bunsen and physicist's Kirchhoff made it clear that the flame color which was characteristic of the metal originated in the emission spectrum of the element. As for this discovery, became the first step of method development to emission spectroanalysis. After that, they discovered rubidium by using the relations between the element and the wavelength in 1860. Cesium was discovered in the same method in 1861.
So the flame reaction can be said as an early method of the emission spectroanalysis.
The structure of the flame reaction
The stability condition of atom that it is filled with the electron one after another from the inner-shell orbital. We say that the ground-state. On the other hand, an inner-shell electron transfer in outer-shell orbital due to some action, and a space is made in inner-shell orbital. We say that the excited-state.
Only un-continuous energy can be taken so that an atom may become the excited-state. Have a characteristic energy level by the material, and the spectrum which is characteristic of the material is decided to be caused as this result.
The flame reaction observe the flame color, when an atom return from the excited-state by the action of flame to the ground-state.
A spectrum exists in the plural because a "characteristic atomic energy level" isn't limited to one and it exists in the plural, too.
Advantage by the movie
The flame reaction has been known since the old days. Only the confirmation of the color is sufficient in the photograph.
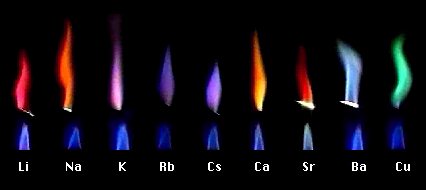
The movie of the flame reaction is seen by using the Internet. It clicks on each flame image of figure1, and open each movie. Then, it can be observed repeatedly how the chlorination thing of each metal element burns on platinum line.
But, actual combustion is early, and it is finished at a moment. So, it was devised that it could play slowly by click the screen of each movie. The state of the combustion can be observed repeatedly by the slow motion by clicking the screen of the movie. If it clicks on the play button of the controller after click a stop button, it becomes play at an actual speed again.
The spectrum image of each flame reaction is indicated on the right of the movie. This is the image observed with the spectroscope in the high school. Generally we observe the flame reaction with the naked eye. But, it can be done clearly the difference in the color that it is observed to the spectrum.
Slit of the spectroscope is changed by the materials. Moreover, the mark that a wavelength is shown in the approximate position, and it isn't accurate. The spectrum image of the sunbeam is indicated to compare it with the spectrum image of the flame reaction.
(It require Quicktime of Apple Computer, Inc.)
How to make an experiment
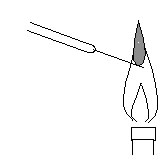
Put each solution of the following concentration on platinum line, and put it in the flame(figure 2) .
1% NaCl
1% KCl
2% RbCl
2% CsCl
1% CaCl2
1% SrCl2
2% BaCl2
10% CuCl2・2H2O
A microscope television device was connected with the spectroscope, and it took pictures as a video movie(figure 3) . After that, each image was kept with the JPEG form.

Spectroscope SHIMADZU KB-2
Microscope television device Toshiba Corp. IK-1590N
Reference book
New chemical library "Bunkoubunsekikagaku" T.Sawada others Dainihontosho Corp. (1988)
Kagakudaijiten Kyoritz Corp. publishing(1963)