The Program Calculating Density and Thermodynamic Properties by the Equations-of-State for Water, Methanol, and Carbon Dioxide
Copyright (c) 2002- Tsutomu Ohmori
Introduction
Brief Overview
Hardware Requirements, Install, Uninstall
Details of the Main Window
How to Use
How to Make Tables of TYPE I (Matrix Type)
How to Make Tables of TYPE II (3 Columns Type)
Restrictions
FAQ (assumed)
Contact the Author
References
Copyright and Exemption Clause, Acknowledgements
2004/10/17
Tsutomu Ohmori
http://hp.vector.co.jp/authors/VA030090/
(The latest information is shown in the above website)
(Old and New information was reflected at Oct. 9, 2010... Of course, English remains poor! )
<<Introduction>>
EOS-SCx is the freeware which calculates density, pressure, and other thermodynamic properties by the equations-of-state for water, methanol, and carbon dioxide, applicable to the wide range of gas, liquid, and supercritical state.
If you study chemistry, you know the equation-of-state (EOS) of ideal gas.
When gas is compressed, its density becomes higher, and at last gas turns liquid usually via the co-existence state.
The EOS of ideal gas cannot be applied to this case.
Indeed, empirical EOS of important substances have been suggested.
I studied the supercritical fluids in the doctoral program.
In this study, the estimation of the density is very important.
When the density is estimated, the empirical EOS based on many experimental data are useful.
However, these EOS are too complicated for the pocket calculator.
Not a few researchers use the tabulated values or their interpolations.
If the precise values of EOS are needed, the calculation programs must be developed.
For several unfortunate reasons, I needed it.
Then, I made a prototype of this program, which is only based on the EOS of methanol.
I need only the density of methanol.
EOS brings out the various properties, e.g. Hermhortz free energy.
Soon after that, I enabled this program to calculate such properties.
This program supports my first published paper very much.
On the other hand, I was exhausted for the development of this program.
So, I think that I should be paid for it.
This payment is "RELEASE as a freeware." for use in various studies.
However, the studies of supercritical methanol are not so many.
Those of supercritical water and carbon dioxide have been reported enormously.
Therefore, we add the EOS of water and carbon dioxide to the program.
Finally, the program has evolved EOS-SCx.
Unfortunately, when the development was completed, I found the calculation programs for the generic equation-of-state: "PROPATH" and the program authored by the group of Wagner, which supports much more substances!
However, these have not prevailed even in the researchers.
At least, I do not find the place for downloading freely.
And anyway, I made it.
Surely EOS-SCx supports your studies at the most, and I hope that.
Back to the TOP
<<Brief Overview>>
This program calculates the density from the temperature and the pressure,
and the pressure from the temperature and the density.
Furthermore, the following thermodynamic properties can be calculated from the EOS:
Melting Pressure (for temperature, without water)
Saturation Pressure (for temperature)
Saturation Density (for temperature)
Internal Energy
Enthalpy
Helmhortz Free Energy
Gibbs Free Energy
Entropy
Isochoric Specific Heat
Isobaric Specific Heat
Compression Factor
Fugacity Coefficient
Sound Velocity
Joule-Thomson Coefficient
Second Virial Coefficient
Isobaric Expansion Coefficient
Isothermal Compression Coefficient
The following properties can be calculated, not from EOS, but from their respective empirical equations:
Viscosity (for water and carbon dioxide)
Thermal Conductivity (for water and carbon dioxide)
Thermal Diffusivity (for water and carbon dioxide)
Relative Dielectric Constant (only for water)
Ion Product (only for water)
Refractive Index (only for water)
Moreover, the above properties can be calculated at many state points automatically and then be tabulated.
In addition, the above thermodynamic properties can be converted between in various units, which you sometimes feel troublesome.
That is how I recommend the EOS-SCx!
Back to the TOP
<<Hardware Requirements>>
EOS-SCx works on Windows OS after Windows95
(I confirmed working on Windows Me, 2000, XP)
The display resolution more than 800*600 is recommended.
Any runtime is not needed.
<<INSTALL>>
Expand EOS-SCx[.zip] (which is compressed) in a folder as you like.
If you are about to ask "how I expand EOS-SCx[.zip]", you should search that in Google or Yahoo.
EOS-SCx never uses a kind of runtime or dll.
<<UNINSTALL>>
Delete the files simply.
Back to the TOP
<<Details of the Main Window>>
Click the parts of the Main Window, and you find the explanations of them.
(Perhaps you cannot jump to the explanation due to your browser.)
(Although the old version is displayed, almost all operations are same as Ver.0.2w.)
Select Fluid
water, methanol, or carbon dioxide can be selected.
Back
T
"T" means Temperature.
The value is input in the left text area and the unit is selected in the right combobox.
K, degC (= degree Celsius), or Tr (= reduced by the critical temperature) can be selected as the unit of the temperature.
When the unit is changed, the values are converted automatically.
Back
P
"P" means Pressure.
For inputting the value, click the left radiobutton.
The value is input in the left text area and the unit is selected in the right combobox.
MPa, Pa, bar, atm (= atmosphere), or Pr (reduced by the critical pressure) can be selected as the unit of the pressure.
When the unit is changed, the values are converted automatically.
In addition, the calculation result of the pressure is displayed.
Back
rho (V)
"rho" means Density. rho<->V Button replaces it for Specific Volume (V).
For inputting the value, click the left radiobutton.
The value is input in the left text area and the unit is selected in the right combobox.
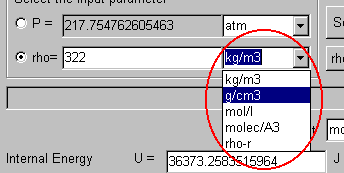
kg/m3, g/cm3, mol/l, molec/A3 (A = Å = angstrom = 0.1nm), or rho-r (reduced by the critical density) can be selected as the unit of the density.
As the unit of the specific volume, the inverses of the above units or Vr (reduced by the critical specific volume) can be selected.
When the unit is changed, the values are converted automatically.
In addition, the calculation result of the density (or specific volume) is displayed.
Back
Set CP
Set the critical temperature (CP) of the selected fluid.
Back
rho<->V
Switch the use of the density (rho) or specific volume (V).
Back
(Error Message Area, no titled)
Error messages are displayed if errors occur.
Back
Calculate
Calculation is performed with the input parameters.
Back
Make Tables
Display the Tabulation Window
Back
About...
Display the references and the information of EOS-SCx.
Back
P-melt
Melting Pressure for the input temperature.
Back
P-satu
Saturation Pressure for the input temperature.
Back
rho-gas (V-gas)
Saturation Density (Specific Volume) of the gas-phase for the input temperature.
Back
rho-liq (V-liq)
Saturation Density (Specific Volume) of the liquid-phase for the input temperature.
Back
Unit of Amount
Select the unit of amount for displaying the values of energy, specific heat, and so on.
mol, kg, or molec (= 1 molecule) can be selected as the unit.
Corresponding units of energy are J/mol, J/kg, and kB/molec (kB = Boltzmann constant = 1.3806e-23 J/K), respectively.
In the selection of molec, the displayed values mean the temperature expression of the energy.
When the unit is changed, the values are converted automatically.
Back
Internal Energy U
It is defined as the sum of the heat transfer into a system and the work done by the system.
It is the free energy of the isochoric system with the variable temperature.
Back
Enthalpy H
It is the free energy of the isobaric system with the variable temperature. H=U+PV.
Notice that this value is not related to enthalpy of formation.
Back
Helmhortz Energy F
It means "Helmhortz Free Energy."
It is the free energy of the isochoric and isothermal system. F=U-TS.
Back
Gibbs Energy G
It means "Gibbs Free Energy."
It is the free energy of the isobaric and isothermal system. G=U-TS+PV.
Back
Entropy S
It is the quantity which is associated with irreversibllity. It is defined as the path integral of the heat transfer into the system divided by the temperature.
It is also defined as the product of the Boltzmann constant and the logarithm of the number of possible microstates of the system in the statistics, so generally interpreted as "the extent of disorer."
Back
Isochoric Spec. Heat Cv
It means "Isochoric Specific Heat." It is the energy for increasing by 1 K on the isochoric system.
Back
Isobaric Spec. Heat Cp
It means "Isobaric Specific Heat." It is the energy for increasing by 1 K on the isobaric system.
Back
Compression Factor Z
Z=PV/RT (R is the gas constant), which shows the discrepancy from the ideal gas.
Back
Fugacity Coef. F/P
For considering the chemical potential, it shows the discrepancy from the ideal gas.
Back
Sound Velocity W
It is the sound velocity in the low-frequency limit.
Back
Joule-Thomson Coef. m
It is defined as the derivative of the temperature by the pressure in the Joule-Thomson process (isenthalpic process). It takes zero in the case of the ideal gas.
Back
Second Virial Coef. C2
It is the second term of the Taylor expansion (Virial expansion) of the compression factor Z. It depends only on the temperature.
Back
Isobaric Expansion Coef. alpha
It is defined as (dV/dT)/V.
Back
Isothermal Compression Coef. kappa
It is defined as -(dV/dp)/V.
Back
Viscosity etha
Back
Thermal Conductivity lambda
Back
Thermal Diffusivity Dth
It is defined as lambda/(rho*Cp).
Back
Relative Dielectric Const. eps
Back
Ion Product pKw
Back
Refractive Index n(l)
It depends on the wavelength.
Back
Back to the TOP
<<How to Use>>
Execute eos-scx[.exe], or, double-click the icon of eos-scx[.exe] or a corresponding operation.
The following window appears.
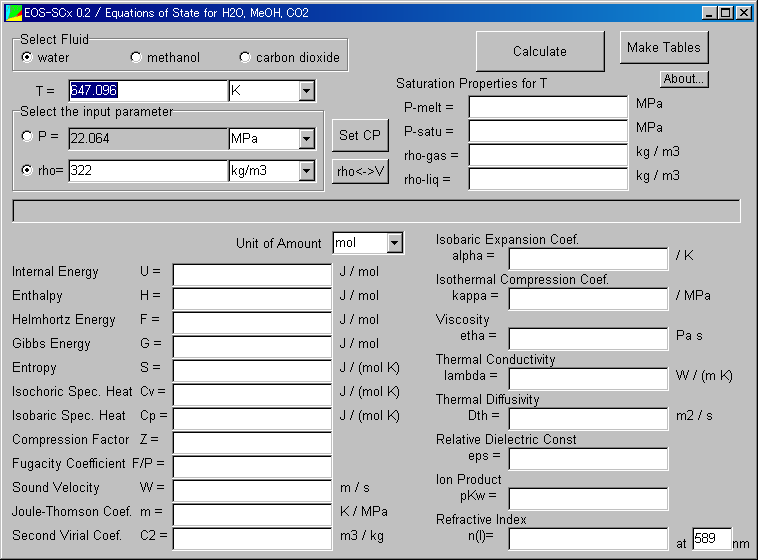
At first, anyway, click the "Calculate."
Then, the window shows the various values.
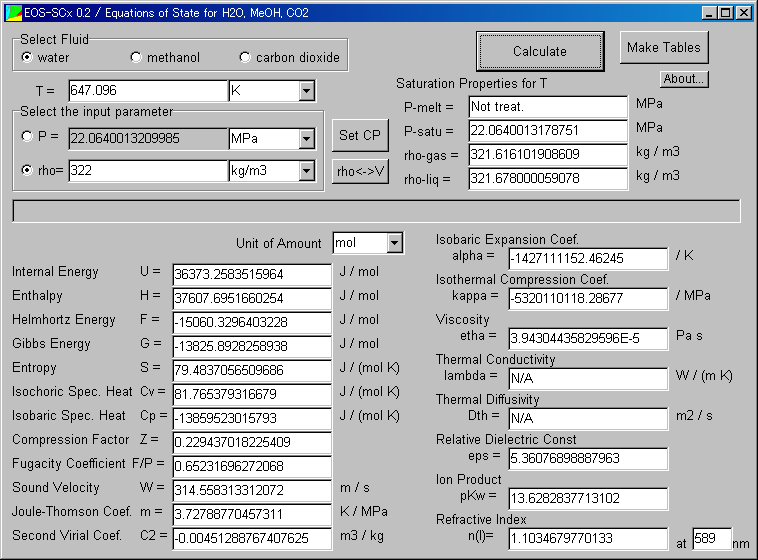
Probably the values appear in a flash.
For calculation, all you do is only the click "Calculate."
Now you perform the calculation at the critical point of water (647.096K, 322kg/m3).
Perhaps you may suspect the value is incorrect. (This reason is mentioned in restrictions)
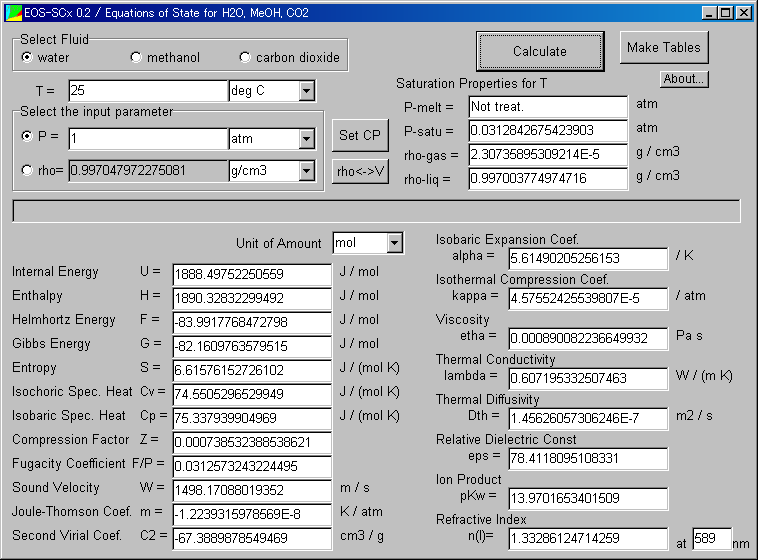
Next, let's calculate the density at the more familiar state point, at 25 degC and 1 atm.
At first you should select the fluid.
But now you do not need change it because the default selection is "water."
Next, select the units.
The unit of the temperature is "degC". Select it.
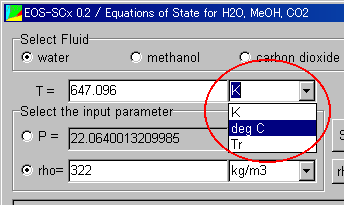
The unit of the pressure is "atm". Select it.
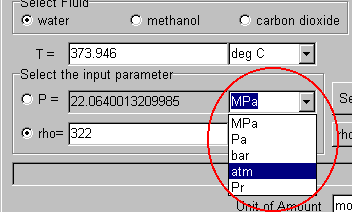
Here, select "g/cm3" as the unit of the density.
The value of the temperature is 25. Input it.
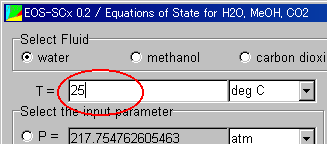
Now, you cannot input the value of the pressure, because the text area is off.
Click the radiobutton on the left side, and the text area turns on and you can input the value of the pressure.
The value of the temperature is 1. Input it.
Now, you finished the preparation. Click "Calculate."

The results are obtained.

For example, 0.99705g/cm3 is obtained as the density value.
It is less than 1g/cm3 you familiar. The precise experimental value is 0.99704g/cm3, which is very close.
By the way, how about the carbon dioxide at the same state point, 25degC and 1atm?
Select "carbon dioxide" for calculating it.
As for the rest, only click "Calculate."

The results are shown below.
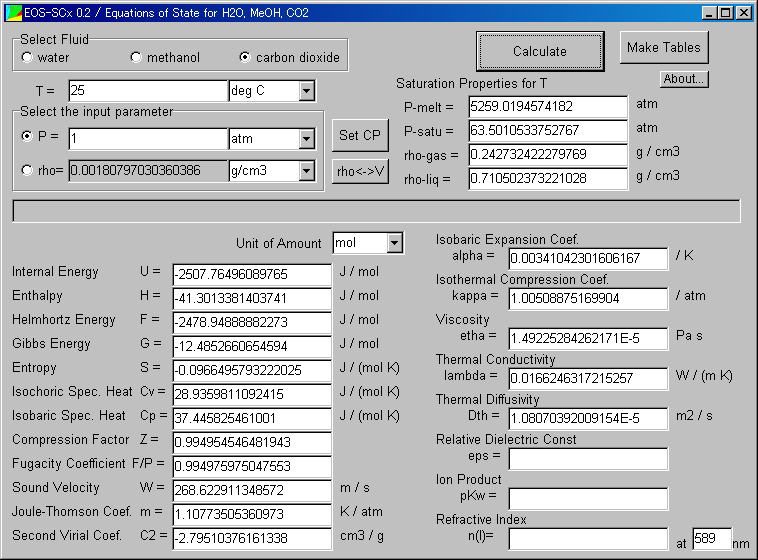
For example, the obtained density value is 0.0018g/cm3, which is very small.
You find it is gas state easily.
Back to the TOP
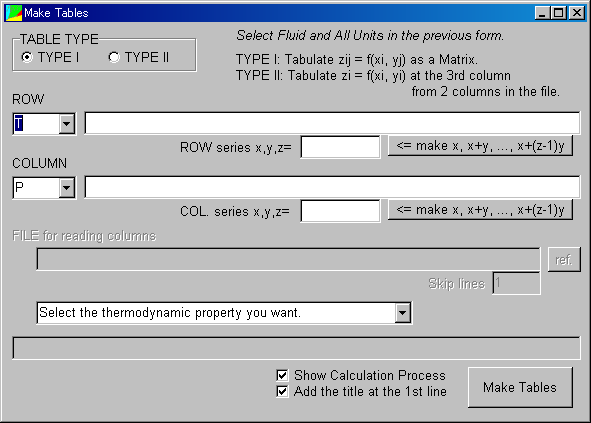
<<How to Make Tables of TYPE I (Matrix Type)>>
A TYPE I table mean an "m*n matrix".
How to make tables of TYPE II is here.
Click "Make Tables," the following window appears.
The default selection is TYPE I in the TABLE TYPE.
Now let's calculate the density of water at 0, 100, 200, and 300 degC and the various pressures from 1 to 100 atm.
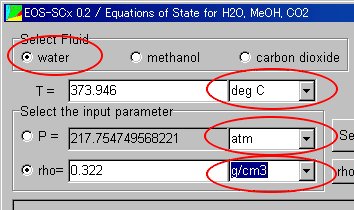
Select the fluid and the units in the Main Window, before operation of the above window.
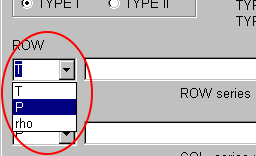
Next, select the quantities of ROW and COLUMN in the table of the matrix type.
You do not need the change the default selection; the temperature and pressure are still selected.
However, the table is easy to be treated when the ROW is pressure and the COLUMN is temperature, because the number of the pressure values is larger.
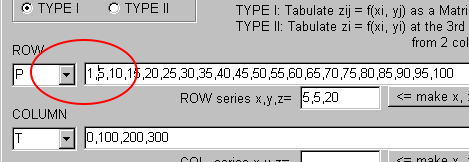
Select "P" (Pressure) in the ROW combobox.
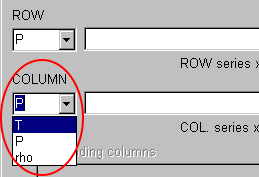
Next, select "T" (Temperature) in the COLUMN combobox.
Here, input the respective values as the comma(",")-delimited text.
For example, in the COLUMN text area, input the temperature values as "0,100,200,300" without spacing.
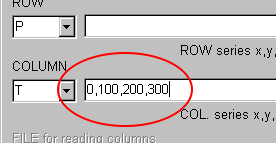
Input the pressure values as "5,10,15,20,25,30,35,40,45,50,55,60,65,70,75,80,85,90,95,100" without spacing, too.
Too long? Yes. This program supports you inputting the many values.
Anyway, input "5,5,20" in the text area on the right side of "ROW series x, y, z=."
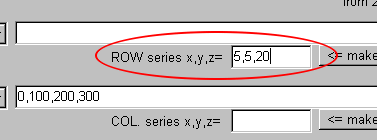
Then, click "<= make x, x+y, ..., x+(z-1)y."
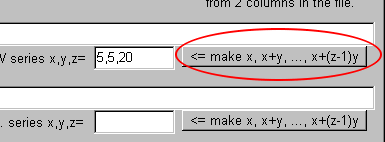
You find the comma-delimited text is created: "5,10,15,20,25,30,35,40,45,50,55,60,65,70,75,80,85,90,95,100"
The input of "5,5,20" means the creation of the series "from 5, increasing every 5, and 20 values."
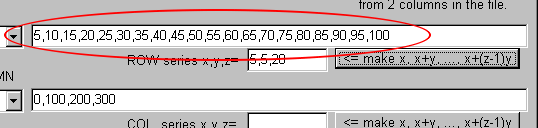
Now add "1," of the head of the series for the calculation at 1atm.
This kind of alteration (addition or deletion) is valid.
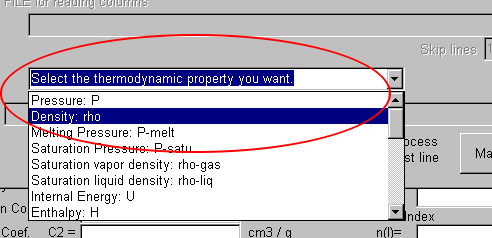
In the below combobox, "Select the thermodynamics you want."
Here, select "Density: rho."
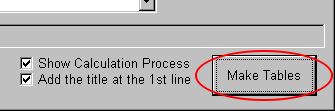
Click "Make Tables."
Then, open the save dialog for the table (in your OS language; below in a typical Japanese dialog).
The table is saved as a text file.
Move to the folder as you want to locate and name the file as you like.
Here, name "test" for example.
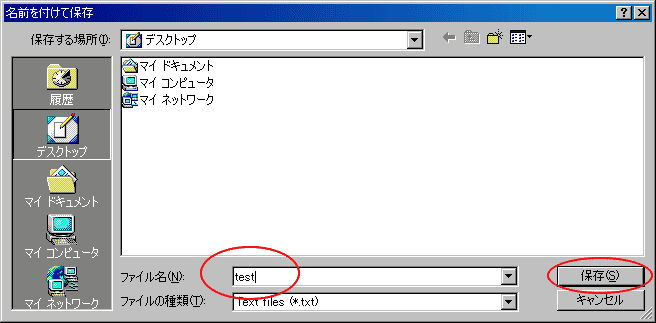
After you name it, click "save" (or corresponding button in your language).
Then, the calculation begins and the calculation process is shown in the Main Window behind.
If you do not want to show the process, check off "Show Calculation Process," in advance.
Check off, and the calculation process becomes faster a little but silent as if it was frozen. You need tolerance to wait a little.
When the calculation is completed, the below message appears.
Look in the table you have made.

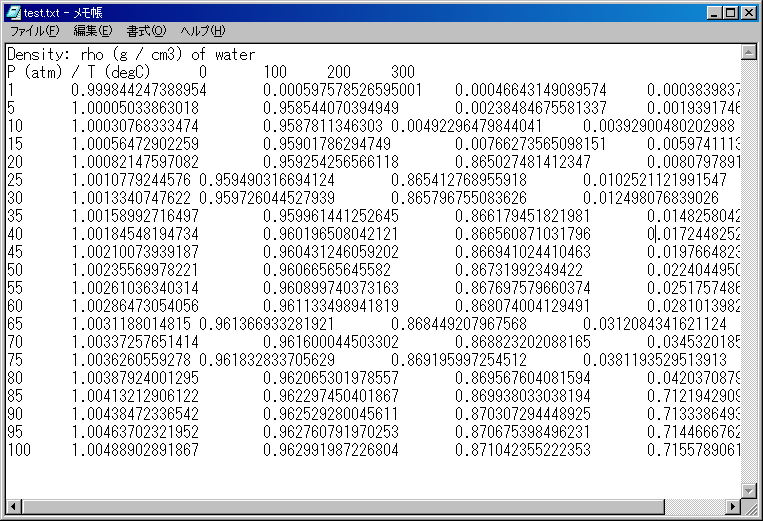
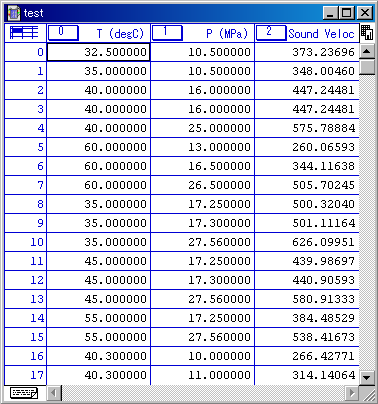
The appearance is the below by the Notepad.
You may think the program created a little bad table, but the output is right.
This table is tab-delimited text.
Now, this table is titled. If you do not need such a title, check off "Add the title at the 1st line," in advance.
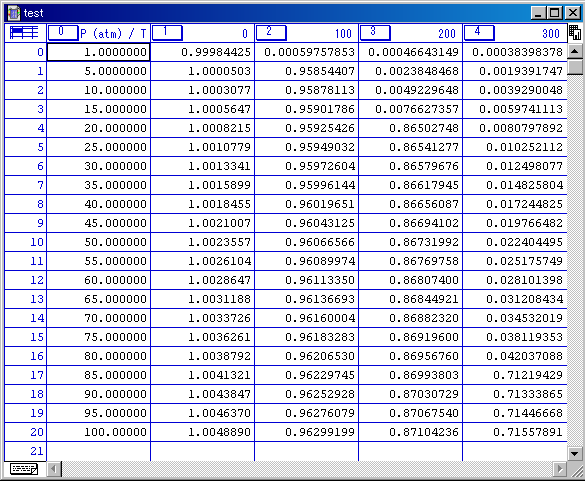
The tab-delimited text is useful for reading the software for analysis the data.
This data can be loaded and justified in such software. (The below shot is Kalaida Graph)
Back to the TOP
<<How to Make Tables of TYPE II (3 Columns Type)>>
A TYPE II table means an "n*3 matrix," which is derived from the "n*2 matrices" in a text file; the element (n, 3) is calculated from the elements (n, 1) and (n, 2).
Therefore, you have to prepare the tab-delimited n*2 matrix as a text file, in advance.
How to make tables of TYPE I is here.
Click "Make Tables," the following window appears.

Select TYPE II in the "TABLE TYPE", the window is switched to be displayed.
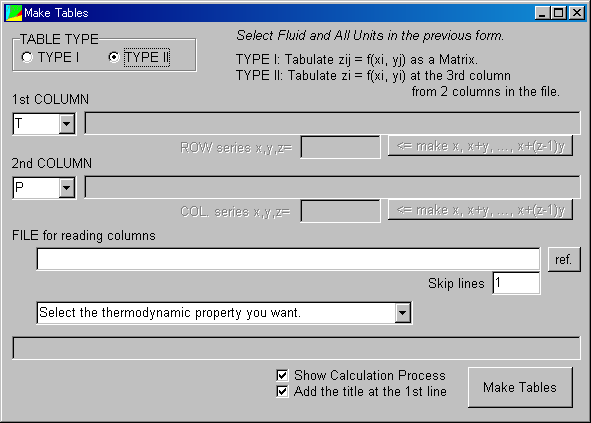
The tab-delimited n*2 matrix as a text file is needed for the following operation.
There is a sample text file is in the package of EOS-SCx: "t2_samp.txt"
The content of t2_samp.txt is below.
Now you use it for creating a TYPE II table.
(By the way, this file was used for the analysis of a certain paper in my study.)
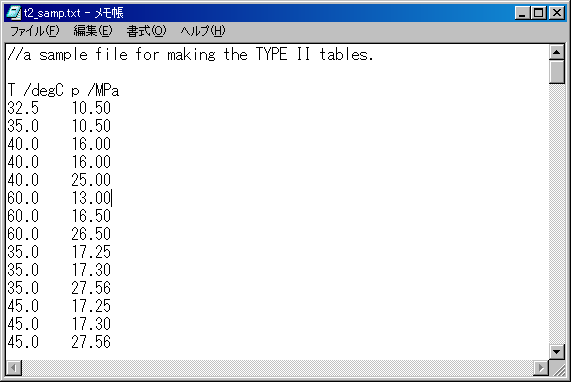
Select the fluid and the units in the Main Window, before operation of the window.
These sample data are used in the analysis of carbon dioxide in the units of degC and MPa.
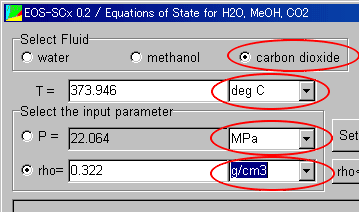
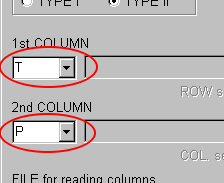
The first column of the sample data shows temperature and the second column shows pressure.
You should select "T" in the 1st COLUMN combobox and "P" in the 2nd COLUMN combobox, but they are same as the default selection.
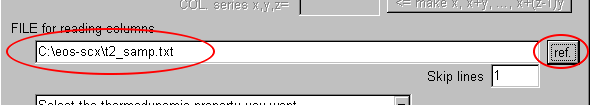
Input the filename (now, "t2_samp.txt") with its full path in the text area of "FILE for reading columns."
Otherwise you can search and set the filename with its full path by clicking "ref."
(The character may be wrong encoded in the languages with 2-byte font, but the problem is probably only the appearance.)
Look at the sample text (t2_samp.txt), and you find the tab-delimited text from the 4th line.
This program can load only a tab-delimited text and must skip 3 lines of this file.
So, input "3" in the text area of "Skip lines."
In the below combobox, "Select the thermodynamics you want."
Here, select "Sound Velocity: W."
Click "Make Tables."

Then, open the save dialog for the table (in your OS language; below in a typical Japanese dialog).
The table is saved as a text file.
Move to the folder as you want to locate and name the file as you like.
Here, name "test" for example.

After you name it, click "save" (or corresponding button in your language).
Then, the calculation begins and the calculation process is shown in the Main Window behind.
If you do not want to show the process, check off "Show Calculation Process," in advance.
Check off, and the calculation process becomes faster a little but silent as if it was frozen. You need tolerance to wait a little.
When the calculation is completed, the below message appears.
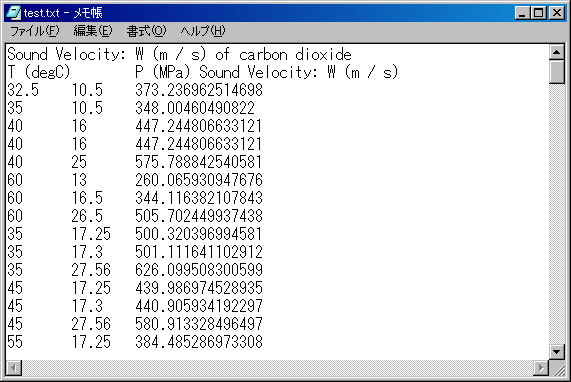
Look in the table you have made.

The appearance is like the below by the Notepad.
This table is tab-delimited text.
Now, this table is titled. If you do not need such a title, check off "Add the title at the 1st line," in advance.
The tab-delimited text is useful for reading the software for analysis the data.
This data can be loaded and justified in such software. (The below shot is Kalaida Graph)
Back to the TOP
<<Restrictions>>
Significant Digits and Errors
This program does not care about the significant digits (as you can see clearly!).
You remember that the significant digits are not more than 6 digits (for carbon dioxide, 5 digits) at most.
In addition, the respective EOS have estimated errors.
The details are in the references.
The Vicinity of the Critical Point
The EOS for methanol itself has the faults in the vicinity of the critical point.
For other EOS, the error becomes larger due to the fated critical behavior in the vicinity of the critical point. In addition, the calculation results are sometimes unlikely, e.g. negative values.
The operation errors are excluded as much as possible, but they may occur.
Calculation from a Pair of Pressure and Density, or from Others.
Beyond this program.
The reference of water suggests the backward equations. If you like, use it.
Meta-stable State
This program gives the values between gas-liquid saturation densities, which is only realized in the meta-stable state, e.g. the supercooled state.
In this case, this program shows the error message as "(meta-stable?)." Although such calculated values are simply extrapolated values, these are adjacent to the "true" values in principle. Reliability of these values should be evaluated.according to the situation.
Notce that such a message pass through in a flash and cannot be recognized when the table is made.
Extrapolated Values out of the Applicable Region of EOS.
The calculation is not performed out of the applicable range of EOS.
The reason is here.
If you need it, you can make a table and extrapolate it.
Consistency of the Table
Even if the errors occur, tabulation often continues.
So the table sometimes includes inappropriate values for the errors.
The Viscosity of Carbon Dioxide in the Gas Phase
In a part of gas density region, the calculation values are wrong for the numerical errors.
So, this program shows the linearly interpolated values between zero density and 5kg/m3.
The Evaluation of the Critical Enhancement for the Viscosity of Carbon Dioxide
1. One of terms of the critical enhancement is neglected because it gives large disagreement with the reference. Calculated values without this term are very good agreement with the reference. Therefore, the term remains neglected. This reason has not been clarified, maybe bugs.
2. Critical enhancement of viscosity is evaluated by solutions of the cubic equation. When state point is very near the critical points, the solutions are triple roots and the critical enhancement cannot be evaluated. Then, the viscosity value without the critical enhancement is shown in bracket beside "N/A." However, the critical enhancement of the viscosity is not large generally. Therefore, this viscosity values in bracket is not so bad in some cases. In addition, critical enhancement of thermal conductivity influences this problem, which is detailed in the following.
The Evaluation of the Critical Enhancement for the Thermal Conductivity of Carbon Dioxide
1. Imaginary number appears in the evaluation. But the imaginary term is neglected finally. The critical enhancement of thermal conductivity is evaluated by the solutions of the quartic equation. Not all solutions are real, but the reference did not explain the treatment of these complex solutions. However, the imaginary part is much smaller than the real part. Although the real part of the calculated values is picked up, the calculated values are good agreement with the reference.
2. Critical enhancement of thermal conductivity is evaluated by using the viscosity value. Described previously, the critical enhancement of viscosity cannot be evaluated in the vicinity of the critical point. Therefore, the viscosity value without the critical enhancement is influenced in the evaluation of the critical enhancement of thermal conductivity. However, the critical enhancement of thermal conductivity is generally large and its precision is poor in the vicinity of the critical point where the critical enhancement of viscosity cannot be evaluated. Therefore, this influence is thought to be small.
Back to the TOP
<<FAQ (assumed)>>
There are several responses, but not "frequently."
So, the following frequently asking questions are "assumed."
Q. It does not work.
When this program is stopped in your operations?
At first, you should make sure of Hardware Requirements, Install, Uninstall.
When you cannot expand the downloaded file, you should make sure of the file size. If the size is different from the information of the website, download it again.
The downloaded file is zip file, which is compressed. Never execute it.
Execute the files eos-scx[.exe] with this icon
If you do not know "compress" or "expand", you should search that in Google or Yahoo.
If you have still problems, it may be bugs. Contact the author as soon as possible.
Q. The calculated values are very different from the references.
Do you select the fluid or units correctly?
If your problem is a kind of the restrictions, it cannot be fixed sooner, sorry.
If you have still problems, it may be bugs. Contact the author as soon as possible.
Q. The calculated values are very different from the references. For example, enthalpy of carbon dioxide is -393.51 kJ/mol at room temperature and atmospheric pressure.
This is "enthalpy of formation (combustion)," which is related in the reaction C(solid) + O2(gas) = CO2(gas) + 393.51kJ/mol. The enthalpy in this program is thermophysical quantity defined as the sum of internal energy U and work done to the system PV, which is related in the phase diagram.
Q. The calculated values are a little different from the references.
Do you compare the calculated values with the experimental values? This is rather reasonably agreement with it in spite of a little discrepancy. You should compare them with the reference of EOS, if you make sure of the validity of this program. According to the restrictions, the significant digits are 6 at most.
For the viscosity and thermal conductivity of carbon dioxide, there is a little discrepancy because the used density values are different from the references.
If you have still problems, it may be bugs. Contact the author as soon as possible.
Q. Little difference was still found despite I input a value of a pressure gauge.
A pressure gauge is usually calibrated as zero point is set under atomasphere. It is called "gauge pressure." So you input an absolute pressure value which is gauge pressure + 1 atm.
Q. Large dfference was still found but temperature dependence and so on are reaonable. So, I think that calculation value is only offset. Is this right?
Yes. Theoretically, energy reaches zero at absolute zero temperature. In usual, a reference value is set and relative values to this are discussed. For example, this program is according to the references. In CO2, enthalpy and entropy of ideal gas are set to zero at 298.15 K and 0.101325MPa as reference values.
Q. Since thermodynamic values are only offset, our reference value is applicable?
Sorry, a reference value cannot be customized.
Q. How do I believe in the calculated values?
Not a little. Otherwise, it depends on the situation.
At least, this program reproduces the values in the table of the references of the EOS, as far as I checked.
So, the reliability of this program is equal to that of the EOS.
Anyway, please compare the calculated values with the values of the references and judge the reliability of this program.
As shown in the restrictions, the incorrect values may be obtained.
Q. What is "N/A"?
"Not applicable."
There are two cases: restrictions in the references or the avoiding the error in this program.
Q. What is "N/A" with a value in bracket for the viscosity of carbon dioxide?
The critical enhancement of the viscosity of carbon dioxide is estimated by the solutions of the third order equation. In the vicinity of the critical point, this estimation is broken because the solution of the equation is the triple root.
The value in bracket shows the viscosity without the contribution of the critical enhancement. However, the critical enhancement of the viscosity is not so large. So, this viscosity values in bracket is not so bad in some cases.
In addition, the thermal conductivity of carbon dioxide is estimated by using the viscosity values. When the viscosity values are in bracket with "N/A," this program calculates the thermal conductivity by using this value in bracket.
In this case, the error is large generally in the vicinity of the critical point. So, I believed that the error is not mainly due to the lack of the critical enhancement of the viscosity.
Q. Why are there blanks in the dielectric constant of carbon dioxide or thermal conductivity of methanol, and so on?
Their experimental values are reported, of course. But the equations estimating them have not been suggested in the wide range.
In the future, when their equations are established, I adopt them.
Q. How is the EOS determined?
I never develop the EOS. I only cited the references.
I think that the methodology is established to some extent for the development of the EOS, and that the experimental data are fitted by the usual functions.
See the references in detail.
If you are interested in the methodology of the development of the EOS, see the following reference.
R. Span, "Multiparameter Equation of State" (Springer-Verlag, Berlin, 2000)
Q. Why is the EOS adopted?
Recommended by IAPWS or IUPAC.
Published in Journal of Physical and Chemical Reference Data.
All parameters of the equations are described in its own paper, not cited.
Validity of the equations is intensively investigated for the comparison with the experimental data.
Tabulated values are described for the verification.
Among the equations satisfying all or some of the above reasons, the newest ones are adopted.
Q. Can the properties of mixture be calculated?
No. These are not simple problems assuming the mixture ratio.
Maybe in the narrower temperature and density ranges, several papers have been reported.
Q. Why are not the extrapolated values shown?
That is the avoiding the responsibility of the authors.
The error messages cannot be noticed necessarily when the values are obtained.
So, the values with fault are used possibly.
The author is most worried about making tables. In this case, the error messages pass thorough in a flash and are not recognized. So, the above possibility of the fault is much larger.
Please give me your opinion for solving the above problem.
Q. The table looks bad.
Tables are consists of the tab-delimited text. So the table is not friendly for the text editors like Notepad.
The software for analysis of data can load such a file and justify the data set, e.g. Excel.
Q. Blanks or messages are outputted in a table.
When the errors occur for inappropriate parameters, this program outputs a blank or an error message.
These results are same as ones obtained by clicking "Calculate" with the respective parameters.
Q. There is only 1 row or column, when a TYPE I table is made. In addition, the table of the pressure and temperature is made although the density and temperature are set.
You select melting pressure, saturation pressure, saturation density, or second virial coefficient, don't you?
These are dependent only on temperature. Pressure or density dependence is meaningless.
So, this program is automatically set the temperature and pressure (the critical pressure is set), when the tables of the above properties are made.
Q. An error occurs in loading a file, when a TYPE II table is made.
Make sure that the text file is a tab-delimited text of an n*2 matrix.
Refer to the sample text (t2_samp.txt).
Make sure whether the incorrect lines are included.
Q. I like to make an enormous table as large as the wallpaper of the room.
The maximum number of row and column is 2048, respectively.
You should connect the divided tables.
Q. This program is opened to the public as a freeware. Is that right? This is based on the papers you cited.
I do not know the copyrights or some related laws very much. But I hear that the copyrights of the binary file, source code and so on are protected for the program.
Because I made this program from scratch with considering the reference coding (not borrowing, at least), no problem is that I claimed that I hold copyrights of this program (which means the copyrights of the source code and so on, to be protected).
In addition, do not misunderstand the meaning of "free." The meaning of "free" means only "free of charge."
Distribution and reproduction are also restricted.
Q. Is this program going to be a shareware?
No. It is because that:
This is prevailed faster and all users are happier.
The payment procedure bothers the users and even the author.
I do not know the appropriate price, although I believe that the value is high.
If so, I have to fix the bugs or compensate the damages by this program, maybe.
If so, strict application of the laws for copyright is demanded.
But I do not reject your chip or contribution!
If you wish to pay it, please contact the author.
Q. Can I distribute this program?
Yes, you can distribute only the original zip archive file without alteration.
In addition, when you distribute it by the website or the mass media, you should request the authorization by contacting the author.
Q. Do this program work on Windows other than Japanese version? You are Japanese.
Several responses have received. Probably this program works.
(The author received first responses from abroad by Dr. Gary Combes, Thanks!)
If not, contact the author as soon as possible.
Q. I want to use this program on Mac or Linux.
You can't. I am sorry for your inconvenient. This program works only on Windows. (Emulator? I do not know it)
I only think that this program will be developed on CUI, with Java, etc., etc..., but these ideas are not realized now.
Back to the TOP
<<Contact the Author>>
According to Exemption Clause, the author is not obliged to compensate the damages by this program.
However, without compensation, the author can do something for you.
Please inquire or tell the author what you want to do, by e-mail in the author's homepage unreservedly, with a common sense, netiquette, and considering the author's exemption from responsibility.
The author is not good at an elaborate work.
There seem bugs I have not found. I would appreciate that you report a bug, and further, find or modify the source code which causes the bug.
The author hopes that this program supports other fluids as many as possible.
If you want to add the equation-of-state for another fluid, please tell your opinion with its reference.
Contact Address
The EOS-SCx website provides the latest program or information.
http://hp.vector.co.jp/authors/VA030090/
Mail address is below.

Tsutomu Ohmori, Ph. D. (Science)
Research Associate (Postdoctral Fellow)
Chemical Spectroscopy Division (Fujii Lab.)
Chemical Resources Laboratory
Tokyo Institute of Technology
Yokohama, 226-8503, Japan
Back to the TOP
<<References>>
[WATER]
W. Wagner and A. Kruse
"Properties of Water and Steam"
(Springer-Verlag, Berlin, 1998)
W. L. Marshall and E. U. Franck
"Ion Product of Water Substance, 0-1000 degC, 1-10000 Bars, New International Formulation and Its Background"
J. Phys. Chem. Ref. Data 10, pp. 295-304 (1981)
[METHANOL]
K. M. de Reuck
"Methanol, International Thermodynamic Tables of the Fluid State Vol. 12"
(Blackwell Scientific Publications, 1993)
[CARBON DIOXIDE]
R. Span and W. Wagner
"A New Equation of State for Carbon Dioxide covering the Fluid Region from the Triple Point Temperature to 1100 K at Pressures up to 800 MPa"
J. Phys. Chem. Ref. Data 25, pp. 1509-1596 (1996)
A. Feenghour, W. A. Wakeham, and V. Vesovic
"The Viscosity of Carbon Dioxide"
J. Phys. Chem. Ref. Data 27, pp. 31-44 (1998)
V. Vesovic, W. A. Wakeham, G. A. Olchowy, J. V. Sengers, J. T. R. Watson, and J. Millat
"The Transport Properties of Carbon Dioxide"
J. Phys. Chem. Ref. Data 19, pp. 763-808 (1990)
Back to the TOP
<<Copyright and Exemption Clause>>
The author Tsutomu Ohmori holds the copyright of this program EOS-SCx and its source code, as is shown in the head of this help and "About..." in the program.
The distribution and reproduction is free regardless of the purpose of profit, as far as you inform me about that in advance.
Only the original archive file of this program without alteration can be distributed or reproduced.
It is free to take out or to change the source code of this program within the bounds of a common sense.
When the program including some or all parts of (altered) source code is sold or distributed, you have to mention that in the manual etc. and have to tell me that.
This program does not completely exclude the possibility of the failure or anything wrong to work.
You can handle this program under the self-responsibility.
If a kind of damage happens due to the use of this program, the author is not obliged to compensate that.
<<Acknowledgement>>
I would like to thank Prof. Kazuyasu Ibuki (Faculty of Engineering, Doshisha University) for browsing the reference of the equation-of-state of methanol.
I also acknowledge Dr. Gary Combes (School of Chemical Engineering and Industrial Chemistry, The University of New South Wales) for checking the work on Windows in (maybe) English.
For the calculation of the solutions of the third and the fourth order equations, I referred to the graduation thesis by Mr. Hitoshi Shinagawa (who graduates Faculty of Engineering, Yokohama National University).
I also referred to the algorithm calculating the inverse matrix by Prof. Shigenobu Aoki (Faculty of Social and Information Technology, Gunma University).
I thanks to them, too.
Back to the TOP